What is H2S gas? What does it smell like and where does it appear?
H2S (Hydrogen Sulfide) is a highly flammable and explosive toxic gas, usually formed in anaerobic conditions where organic compounds are decomposed by microorganisms. In addition to its natural origin, H2S also appears in many industrial activities such as oil and gas exploitation, chemical processing or wastewater treatment. Although it is common, this gas is extremely dangerous if not properly controlled.

Unlike many other toxic gases, H2S has almost no distinct odor at high concentrations, making it difficult for humans to detect leaks. At low levels, it can sometimes have a slight rotten egg odor, but the ability to detect it quickly disappears as the concentration increases because H2S paralyzes the olfactory nerve. Therefore, relying on smell to detect H2S is completely unsafe.
H2S occurs in many different environments, especially where there is an anoxic decomposition of organic matter such as landfills, swamps, wells, oil and gas fields or wastewater treatment areas. In industry, this gas is also generated in paper production, oil refining, food processing and many activities that use sulfur compounds. Whether natural or artificial, the presence of H2S always needs to be closely monitored to ensure safety for humans and the environment.
Is H2S gas toxic?
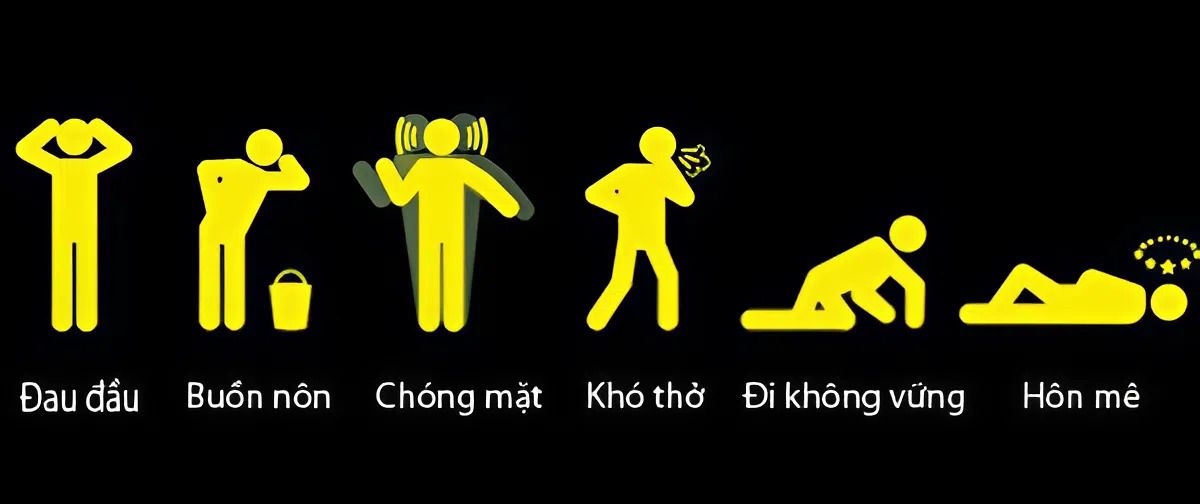
After understanding what H2S is and its basic properties, the next question that is often asked is: is this gas toxic? In fact, H2S is an extremely toxic gas, capable of causing serious effects on human health in a short time if inhaled or directly exposed to the skin or eyes.
When the body is exposed to H2S, people often feel headaches, dizziness, nausea, fatigue and difficulty breathing. At higher levels of exposure, this gas can damage the nervous system, cause movement disorders, reduce reflexes, and even lead to loss of consciousness or death if not treated promptly. In particular, because H2S can paralyze the sense of smell, victims often do not realize its presence until the body reacts violently.
Because of this dangerous nature, H2S is often used as a warning sign for toxic gas leaks in many industries. Sensors, alarm systems and gas monitoring devices are all designed to detect H2S early, helping to protect workers in potentially exposed environments.
How to safely measure H2S gas
To determine the concentration of H2S in the air, people often use a specialized H2S gas meter. This device helps detect the presence of toxic gas and warns when the measurement level exceeds the allowable threshold.
Before measuring, it is necessary to check the power source and calibrate the machine to ensure the sensor is working properly. Then, set the measurement unit and warning threshold according to usage requirements.
When operating, place the device in the area to be tested and keep it stable for a few seconds for the sensor to record the value. The H2S concentration result will be displayed immediately on the screen, can be saved or sent to the monitoring system if the device supports data connection.
Suggestions for H2S gas measuring devices that are trusted today
Multi-gas detector HFP-0401(A)
A popular choice in the toxic gas monitoring group is the HFP-0401(A) multi-gas detector. This model is capable of detecting six different gases at the same time, including CH₄, CO, H₂S, O₂, CO₂, and NH₃. Integrating multiple sensors in the same device makes it easy for users to monitor the working environment, especially in areas where there is a risk of gas mixture leakage.
The device uses electrochemical, catalytic and infrared sensors to detect the concentration of each gas. The wide measuring range, from 0–100% LEL for CH₄, 0–30% volume for O₂, to 0–200 ppm for H₂S and NH₃, allows flexible monitoring in a variety of industrial conditions.
With a clear display and the ability to set alarm thresholds, the HFP-0401(A) helps operators detect risks early and handle them promptly when gas concentrations exceed safe levels.
Refer to some other models such as: INDUSTRIAL SCIENTIFIC MX4, SENKO SP-SGTP O2,... currently available on the EMIN website system





